Abstract
This case report describes a 50-year-old man with a history of hypertension, hyperlipidemia, and poorly controlled diabetes who presented with symptoms of nausea, vomiting, abdominal pain, fevers, and chills. He was found to have group B streptococcal bacteremia, leading to a series of complications, including aortitis secondary to a prevertebral abscess. Despite challenges in management, the patient responded well to antibiotics and anticoagulation therapy, resulting in complete resolution of the prevertebral abscess and aortitis. This case highlights the importance of early recognition and multidisciplinary management in cases of severe group B streptococcal infections.
Introduction
Group B Streptococcus (GBS), or Streptococcus agalactiae, is a leading cause of bacterial infections in neonates and pregnant women. Invasive GBS infections in adults are often associated with underlying conditions such as diabetes, malignancy, and immunosuppression. GBS is a common colonizer of the gastrointestinal and genitourinary tracts and is frequently associated with diabetic skin, soft tissue infections, and urinary tract infections in adults. Endovascular infections related to Group B streptococci are less common, and aortitis is a rare complication that can result in life-threatening consequences if not promptly recognized and managed. The first documented case of a mycotic aneurysm due to Group B Streptococcus was described in 1989 from the direct invasion of an L2/L3 paravertebral abscess and osteomyelitis into an abdominal aortic aneurysm. This case was managed by excision and primary anatomical graft [1]. Eight cases of infected abdominal aortic aneurysms secondary to GBS have been described in the literature and were managed surgically with excision and grafting versus bypass [2]. This case describes an infected abdominal aortic mural thrombus without an aneurysm that was conservatively managed with an extended course of intravenous antibiotics and anticoagulation.
Case Report
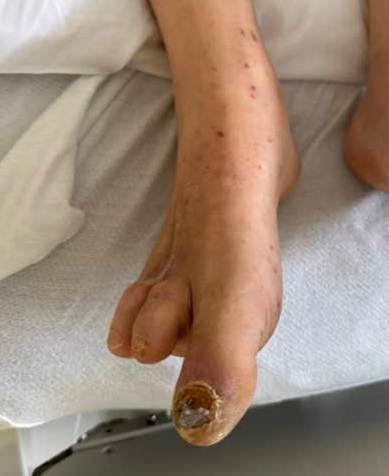
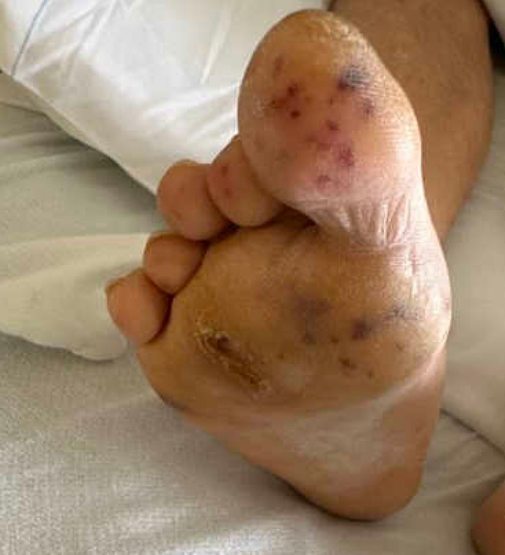
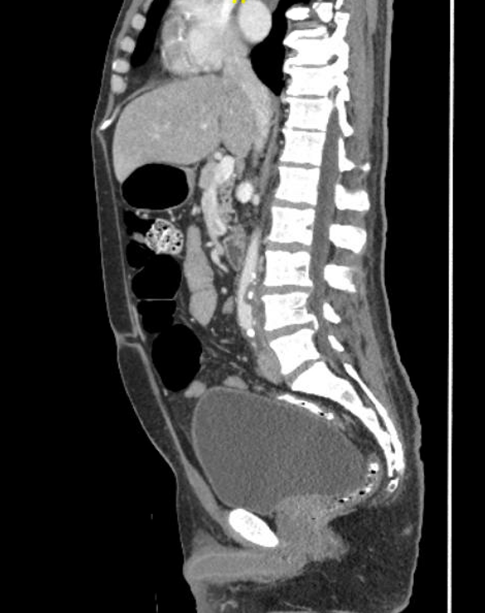
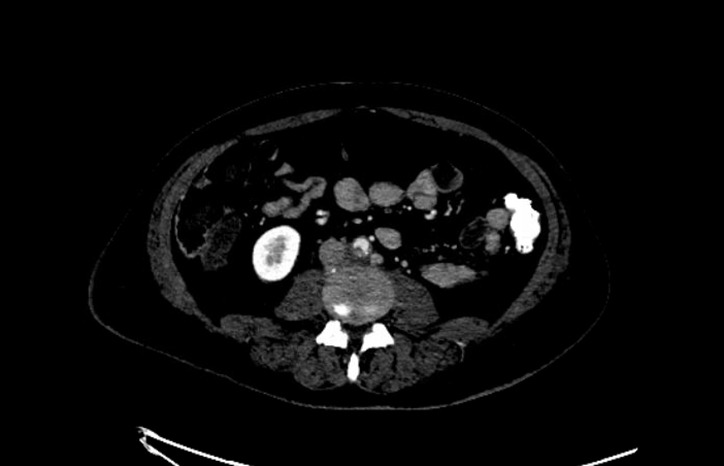
A 50-year-old male with a past medical history of hypertension, dyslipidemia, and poorly controlled type 2 diabetes mellitus presented to the hospital with nausea and vomiting. He described lower abdominal pain and significant pain in his lower extremities. The patient had been intermittently compliant with his insulin and glucose monitoring. His blood glucose level on admission was 396, and his hemoglobin A1C was 14.5%. He denied any significant weight loss, fevers, chills, cough, shortness of breath, back pain, or chest pain. He described “heaviness” and pain in the lower extremities. Physical examination showed a temperature of 36.6 °C, heart rate 78 beat/minute, blood pressure of 138/70 mmHg, and respiratory rate of 17 breaths/minute. He was noted to have a systolic murmur on exam, as well as multiple painful erythematous nodules on the tips of his toes and plantar feet, concerning for embolic phenomena (see Figures 1 and 2). He had strong distal pulses. Complete blood count revealed hemoglobin 15.3 g/dL, WBC 17,900 cells/mm3 (neutrophils 90%), and platelet 1,270,000/mm3. Creatinine was 0.87 mg/dL, and albumin was 2.6 g/dL. His C-reactive protein was 12 mg/dL. Two sets of blood cultures were positive for GBS. A transesophageal echocardiogram revealed no evidence of vegetation. Computed tomography angiograms of his chest, abdomen, and pelvis revealed a 5 by 1 cm prevertebral abscess at the L4 level with an aortic mural thrombus adjacent to, or in continuity with, the prevertebral abscess, resulting in significant moderate luminal narrowing (see Figures 3 and 4). Infectious disease, vascular surgery, neurosurgery and hematology teams were consulted. The patient was started on full-dose anticoagulation with enoxaparin 1 mg/kg dosing every 12 hours. His bacteremia cleared quickly on ceftriaxone. Based on the location of the abscess and the initial improvement with intravenous antibiotics and anticoagulation, both vascular and neurosurgical teams recommended conservative management. The abscess was not accessible for drainage using interventional radiology based on its location. The patient was treated for 3 months using intravenous antibiotics until there was complete resolution of the prevertebral abscess and significant improvement of the focal mural thrombus. Anticoagulation therapy was continued for 6 months. The patient has been followed for over 25 months without any relapse of infection.




Discussion
GBS bacteremia can lead to a range of complications, including endocarditis, pneumonia, meningitis, and sepsis. The ability of GBS to evade host immune responses and invade different body sites can result in disseminated infections. In the case presented, the patient exhibited embolic phenomena, likely due to the hematogenous spread of GBS from the prevertebral abscess to the abdominal aorta, causing aortitis. Reports of aortitis secondary to abutting abscesses are limited in the literature but highlight the potential for serious vascular complications. In the traditional management of endocarditis, anticoagulation has historically been avoided due to early observational studies suggesting an elevated risk of cerebral hemorrhage in patients with endocarditis. However, in our patient’s case, considering the distal aortitis and predominant clot formation, the decision to initiate anticoagulation was beneficial [3]. Microorganisms can gain access to the aorta through contiguous spread from adjacent structures or originate from bacterial endocarditis, commonly affecting the ascending aorta. In non-endocarditis bacteremia, the most commonly affected site is an aneurysm in the abdominal aorta due to atherosclerotic plaque. Notably, in our patient, there was no aneurysm, and this was primarily an infection via contiguous spread. The most common pathogens that cause an infected aneurysm when caused by endocarditis are Streptococcus viridans, Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Haemophilus, and Pneumococcus [4]. When it does not involve bacterial endocarditis but is a primary infected abdominal aortic aneurysm, the most common pathogens are Salmonella spp., Streptococcus, Bacteroides, Escherichia coli, and Staphylococcus aureus. Atypical organisms that can cause complicated endovascular infections include Coxiella burnetti, Bartonella henselae, Bartonella quintana, Brucella species, Tropheryma whippelei, endemic mycoses, and Mycobacterium tb complex [5]. Prompt diagnosis and treatment are crucial to preventing catastrophic outcomes such as aortic dissection or aneurysm formation. This case underscores the importance of a multidisciplinary approach involving infectious diseases, vascular surgery, and hematology in managing complex GBS-related vascular infections.
GBS is a prevalent colonizer of the gastrointestinal and genitourinary tracts. However, it can lead to severe infections, especially in immunocompromised individuals, including the elderly, those with poorly managed diabetes, cirrhosis, and patients with underlying malignancies. GBS is also the most common cause of sepsis in newborns [6]. GBS possesses several virulence factors that contribute to its pathogenicity, including polysaccharide capsule, adhesins, and toxins. The capsular polysaccharide of GBS play a significant role in immune evasion and resistance to phagocytosis [7]. Adhesins enable GBS to adhere to host cells and tissues, facilitating colonization and invasion. Toxins produced by GBS can promote the invasion of host cells and trigger host cell lysis and inflammation, contributing to the severity of invasive infections [8]. Understanding these virulence factors is essential for developing targeted therapeutic strategies and vaccines against GBS infections.
This case report highlights the clinical challenge of managing GBS bacteremia complicated by a prevertebral abscess and aortitis in an adult patient with multiple comorbidities. The patient’s poorly managed diabetes was likely the primary factor contributing to the severity of his infection. Since the COVID-19 pandemic, there has been a noticeable increase in the virulence of streptococcal infections. The extent to which immunity debt also contributed to the severity of this patient’s infection remains uncertain. Fortunately, the patient exhibited a favorable response to a prolonged antibiotic regimen, anticoagulation, and improved glycemic control. Timely diagnosis, appropriate antimicrobial therapy, and interdisciplinary collaboration were essential to achieving a successful patient outcome.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blackett, R.L.; Hill, S.F.; Bowler, I.; Morgan, J.R.; Heard, G.E. Mycotoxins aneurysm of the aorta due to group B Streptococcus (Streptococcus agalactiae). Eur. J. Vasc. Surg. 1989, 3, 177–179. [CrossRef] [PubMed]
- Upapan, P. Mycotic aneurysm of the abdominal aorta due to group B Streptococcus (GBS) infection: A case report and review of the literature. J. Med. Assoc. Thai 2016, 99 (Suppl. 8), S253–S259. [PubMed]
- Randhawa, M.S.; Pile, J.; Gomes, M. Can Patients with infectious endocarditis be safely anticoagulated? Clevel. Clin. J. Med. 2016, 83, 169–171. [CrossRef] [PubMed]
- Stengel, A.; Wolfforth, C.C. Mycotic bacterial aneurysms of intramuscular origin. Arch. Intern. Med. 1923, 31, 527–554. [CrossRef]
- Wouthuyzen-Bakker, M.; van Oosten, M.; Bierman, W.; Winter, R.; Glaudemans, A.; Slart, R.; Toren-Wielema, M.; Tielliu, I.; Zeebregts, C.J.; Prakken, N.H.; et al. Diagnosis and treatment of vascular graft and endograft infections: A structured clinical approach. Int. J. Infect. Dis. 2023, 126, 22–27. [CrossRef] [PubMed]
- Berner, R. Significance, management and prevention of Streptococcus agalactiae infection during the perinatal period. Expert Rev. Anti-Infect. Ther. 2004, 2, 427–437. [CrossRef] [PubMed]
- Marques, M.B.; Kasper, D.L.; Pangburn, M.K.; Wessels, M.R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 1992, 60, 3986–3993. [CrossRef] [PubMed]
- Rajagopal, L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiol. 2009, 4, 201–221. [CrossRef] [PubMed]

