Home » Case Reports » Enigmatic CNS Lupus Vasculitis: A Heuristic Approach
- December 31, 2023
Enigmatic CNS Lupus Vasculitis: A Heuristic Approach
Christopher Szewczyk
Rush Medical College, 600 S Paulina St, Suite 524, Chicago, IL 60612, USA
You can also search for this author inPubMed | Google Scholar |
Hemil Gonzalez, MD
Department of Internal Medicine, Division of Infectious Diseases, Rush University Medical Center, 600 S Paulina St #140, Chicago, IL 60612, USA
Contact Hemil Gonzalez, MD
You can also search for this author inPubMed | Google Scholar |
How to Cite: Szewczyk, C.; Gonzalez, H. Enigmatic CNS Lupus Vasculitis: A Heuristic Approach. Priv. Pract. Infect. Dis., 2023, 3(4): 10; doi:10.55636/ppid3040010.
© 2023 Copyright by Author. Licensed as an open access article using a CC BY 4.0 license.
Abstract
Systemic lupus erythematosus is an autoimmune disease affecting all organs of the body. It has a higher incidence in women and can affect the central nervous system (CNS) in 10% to 20% of cases. Any structure in the CNS can be involved, resulting in a varied and often complex symptomatology. CNS lupus vasculitis is an even rarer manifestation characterized by small vessel inflammation that can result in vessel thrombosis and necrosis. We present the case of a 31-year-old woman with a history of systemic lupus erythematosus and Sjögren’s syndrome who was treated at our institution for an acute exacerbation of lupus complicated by CNS lupus vasculitis. This case was particularly challenging due to concurrent bacteremia and a travel history that broadened the differential diagnosis widely, thus requiring our input as infectious disease consultants. CNS lupus vasculitis is a diagnosis of exclusion that requires a high level of clinical suspicion to arrive at a prompt diagnosis paired with aggressive management to guarantee good outcomes. In this report, we share our stepwise approach to this challenging case in a didactic manner to benefit trainees and independent infectious diseases providers who may encounter similar clinical scenarios in their practice.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by a chronic course with acute exacerbations affecting multiple organ systems [1]. A “textbook” SLE presentationincludes a malar rash, fevers, arthralgias, and positive serologies. However, lupus syndromes are heterogeneous and can overlap significantly, thus delaying a diagnosis [2].
Similarly, the central nervous system (CNS) can be affected as part of a systemic lupus syndrome or in isolation [3]. Nonspecific symptoms can include headaches, fatigue, or depression, while potentially more serious presentations include seizures, strokes, intracranial hemorrhages, and cranial nerve involvement [4]. Myelitis, peripheral neuropathy, and autonomic neuropathy are less common but can lead to significant morbidity [5].
CNS lupus vasculitis (CNSLV) is a rarer presentation in which small vessels and capillaries are affected [6]. Clinical syndromes include meningitis, ischemia (stroke), intracranial hemorrhage, and transient ischemic attacks (TIAs) [7]. CNSLV is mediated by immune complex deposition in terminal vasculature, resulting in endothelial dysfunction, thrombosis, vessel wall damage, and capillary leakage. Antiendothelial cell antibodies are the primary autoantibodies associated with this condition. Still, other antibodies, including antineutrophil cytoplasmic antibodies, antiphospholipid, and anti-double-stranded DNA, have been reported [8]. Their binding can independently induce endothelial cells to upregulate their adhesion molecules and to release cytokines and chemokines, thus recruiting inflammatory cells to the vessel wall.
In addition, these autoantibodies will activate the complement cascade, further exacerbating the pro-inflammatory, prothrombotic, and pro-adhesive effects on endothelial cells. Activation of this inflammatory cascade leads to upregulation of coagulation factor production and results in thrombosis, neutrophil sequestration, and vessel wall necrosis. Peripheral lupus serology is positive in 80% to 100% of patients with CNSLV [8]. However, normal inflammatory markers and quiescent lupus do not exclude underlying CNS vasculitis [3].
Cerebrospinal fluid (CSF) examination does not allow for direct diagnosis of CNSLV but helps exclude infection or other mimickers [9,10]. Furthermore, it can reveal auto-antibodies, though these must be interpreted with caution as they can be present in patients without underlying pathology [8]. The CSF profile will show pleocytosis that is most often mononuclear cell predominant, protein elevation that does not typically exceed 100 mg/dL, and an increased IgG index without oligoclonal bands [11]. CSF glucose levels can be normal or decreased but are usually higher than those found in CNS infections [10,12]. CSF microbial stains and cultures should be negative unless a concurrent infection exists [7,10,11]. The CSF/serum albumin ratio may be altered due to endothelial involvement and disruption of the blood–brain barrier (BBB) [4].
The gold standard for diagnosing CNS lupus vasculitis is a meningeal and brain biopsy, although these are seldom carried out due to the inherent risks and limited sensitivity [6,13,14]. Characteristic pathology findings include leukocytoclastic vasculitis, characterized by diffuse mononuclear cell infiltration across the vessel wall with fibrinoid necrosis, IgG, and complement deposition [11,15]. However, the diagnosis can be made without a biopsy by combining clinical features, appropriate imaging findings, and diagnostic studies. Magnetic resonance imaging (MRI) is the most sensitive noninvasive study for CNSLV [7,16,17]. Although this technique can reveal vessel wall thickening, beaded appearance, and intramural contrast uptake when large- and medium-sized vessels are involved, it cannot, however, detect small-vessel involvement [17,18]. Similarly, small-vessel disease is beyond the resolution of conventional angiography, and a normal angiogram is not uncommon in the presence of biopsy-proven disease [18,19].
Case Description
A 31-year-old female with a past medical history of uncontrolled SLE, Sjögren’s, and anemia of chronic disease presented to the rheumatology clinic with acute chest pain, abdominal pain, and dyspnea. This patient was diagnosed with SLE approximately 9 months before the index hospitalization and was initially treated with prednisone; she later transitioned to hydroxychloroquine and methotrexate. Her most recent regimen was hydroxychloroquine and azathioprine. During the month prior to her visit to the rheumatology clinic, the patient had been nonadherent with her immunosuppressive therapy and pursued naturopathic remedies in her native Ecuador. Since then, she developed oral ulcers leading to poor oral intake, punched-out digital ulcers, fevers, arthralgias on the upper and lower extremities, and left ankle swelling.
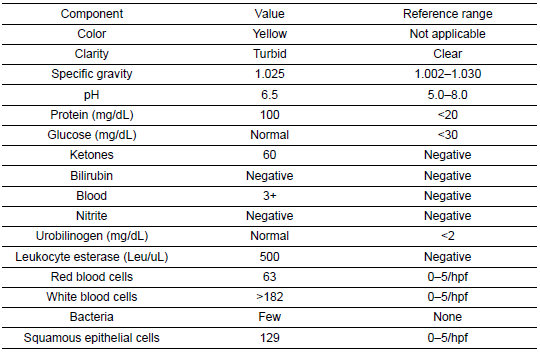
She was subsequently transported from the rheumatology clinic to the Emergency Department with notable vitals of a blood pressure of 90/52, heart rate of 114, temperature of 103.1°F, respiratory rate of 18, and an oxygen saturation of 95% on 2 L of oxygen via nasal cannula. Her initial labs upon admission were significant for hyponatremia, transaminitis, anemia, coagulopathy, elevated creatinine kinase, abnormal markers of SLE disease activity, and hematuria (Tables 1–5). Blood and urine cultures were drawn. An electrocardiogram demonstrated sinus tachycardia but no other abnormalities. Computed tomography scans of the chest, abdomen, and pelvis showed scattered pulmonary nodules measuring up to 0.8 centimeters and located bilaterally, multiple enlarged left axillary lymph nodes, a hepatic cyst, and thickened bladder walls with mucosal ulcerations. There was no evidence of pulmonary embolism, vascular occlusions, dissection, or aneurysms on the echocardiogram or vascular Dopplers. The patient was started on 2 L of Ringer’s lactate, intravenous (IV) ceftriaxone and vancomycin, multiple doses of IV hydromorphone, and 2 g of calcium gluconate.
Table 1: Complete Metabolic Panel
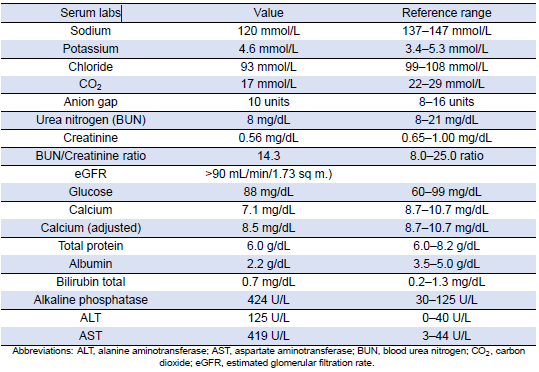
Table 2: Cardiac Labs
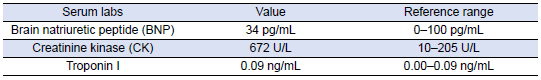
Table 3: Complete Blood Count and Coagulation Parameters
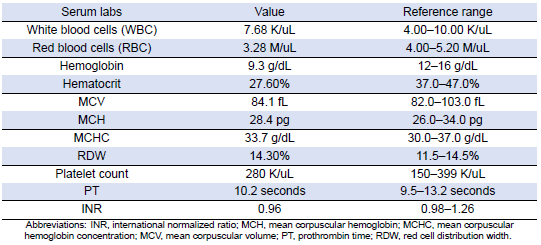
Table 4: Pertinent Autoimmune Markers
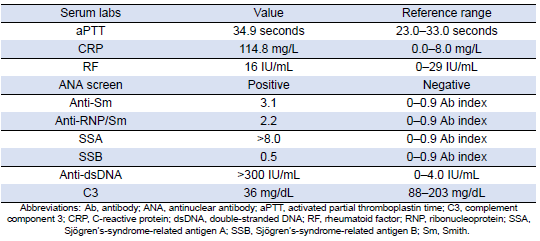
Table 5: Urinalysis
On the second day of admission, blood and urine cultures showed growth of Pseudomonas aeruginosa. Antimicrobials were changed to piperacillin–tazobactam, and the patient was restarted on hydroxychloroquine. Next, cultures were repeated to rule out an acute infectious process before initiating prednisone therapy for suspected SLE exacerbation.
On the third day of admission, she rapidly began to decompensate and became hypotensive with obtundation. Given the concern of septic embolization in the context of bacteremia, she underwent an MRI of the brain, which showed a multitude of foci with restricted diffusion and surrounding edema, as seen in Figure 1.
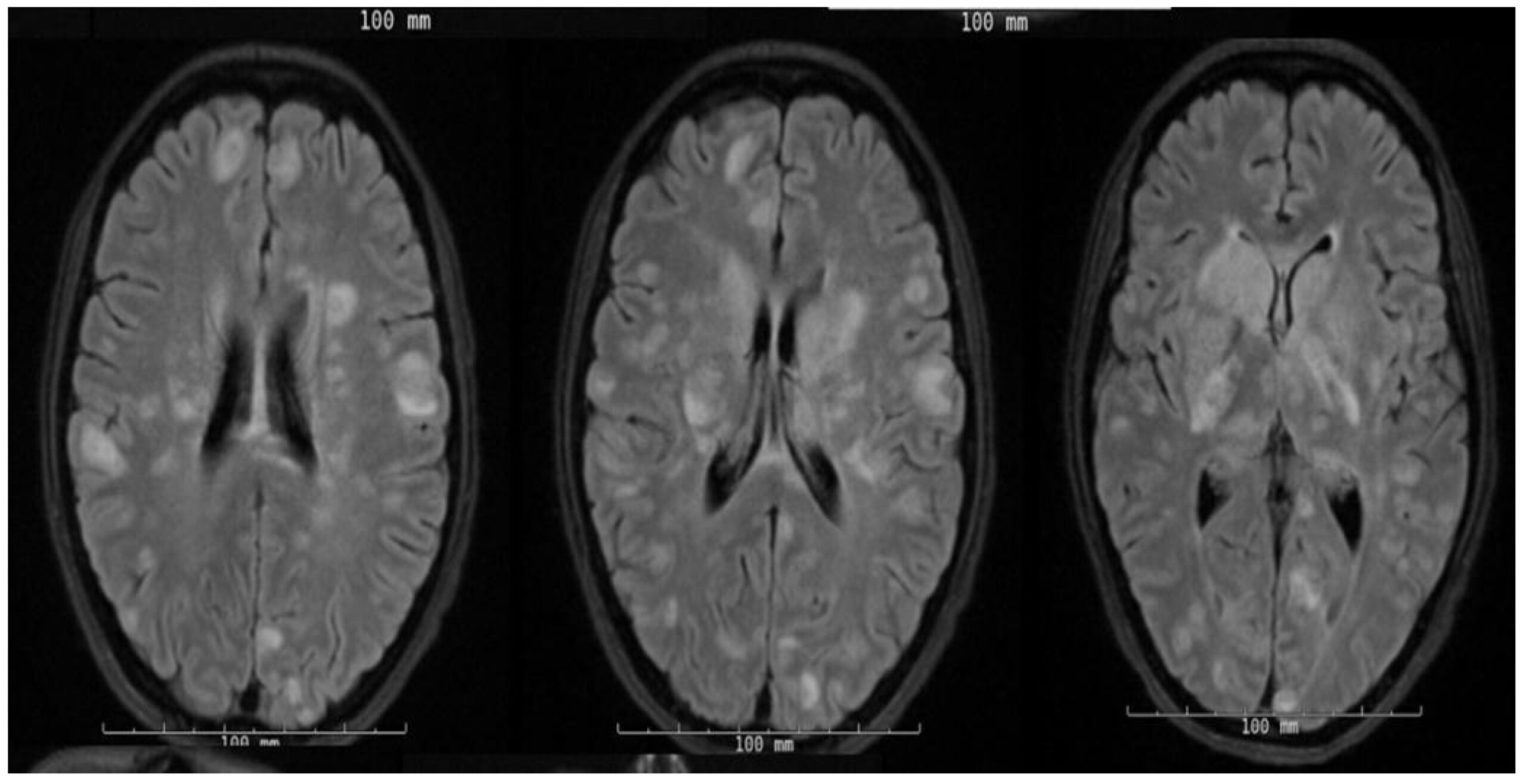
Figure 1: MRI T2 axial flair sequence showing numerous punctate to small foci of restricted diffusion with surrounding edema in the supra and infratentorial brain parenchyma. Few of these lesions demonstrate subtle rim enhancement. Mild mass effect without midline shift or other intracranial herniation. A few scattered micro hemorrhagic foci are observed.
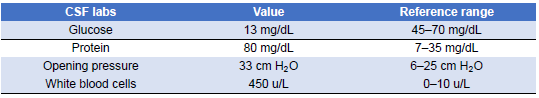
Given this new clinical change and concerning imaging findings, the patient’s antibiotic regimen was changed to vancomycin and meropenem to broaden coverage. At this point in the admission, antimicrobial susceptibilities for the Pseudomonas aeruginosa isolate were not yet available, and it was suspected it could be an extended-spectrum-β-lactamase producer. Although the patient became hemodynamically stable, her altered mentation persisted, and she underwent a lumbar puncture (LP) on the fourth day of admission. CSF findings were significant for white blood cell (WBC) count of 450 u/L (reference range 0–10 u/L), glucose level of 13 mg/dL (reference range 45–70 mg/dL), protein level of 80 mg/dL (reference range 7.0–35.0 mg/dL), and an opening pressure of 33 cm of water (Table 6). The antibiotic regimen was further broadened by adding amphotericin and ampicillin to cover fungal etiologies and Listeria monocytogenes empirically. However, CSF stains showed no organisms, cultures showed no growth, and amphotericin was discontinued. At the time, the possibility of negative CSF cultures due to prior antibiotic therapy was considered, but the overall constellation of findings was still thought less likely to represent infection. Subsequent blood cultures showed no growth. Given this negative infectious workup, it was suspected that the patient’s CNS symptomatology was more likely due to her SLE rather than an infectious cause, and she was placed on a 5-day course of 1 g of intravenous methylprednisolone daily.
Table 6: Initial Cerebrospinal Fluid (CSF) Analysis on Day 4 of Admission
Despite the patient being started on steroids, her neurologic status continued to decline; she could now not follow commands. She was transferred to the medical intensive care unit for supportive care on day 5 of admission. Stroke workup and repeat brain MRI were negative, with no additional changes from prior imaging noted. Computed tomographic angiography (CTA) of the brain was also performed, which demonstrated no abnormalities in the cerebral vasculature (Figure 2). A fluoroscopic angiogram was deferred, given that the patient had developed an acute kidney injury and hyperkalemia, and the yield for small vessel vasculitis would be low. Furthermore, continuous electroencephalogram monitoring demonstrated no epileptiform discharges. Neurology consultation determined that the next best course of action would be to start the patient on a 5-day course of plasmapheresis due to high suspicion of CNSLV. On day 6 of admission, she was started on plasmapheresis treatment of 5 sessions per day every other day for 1 week.
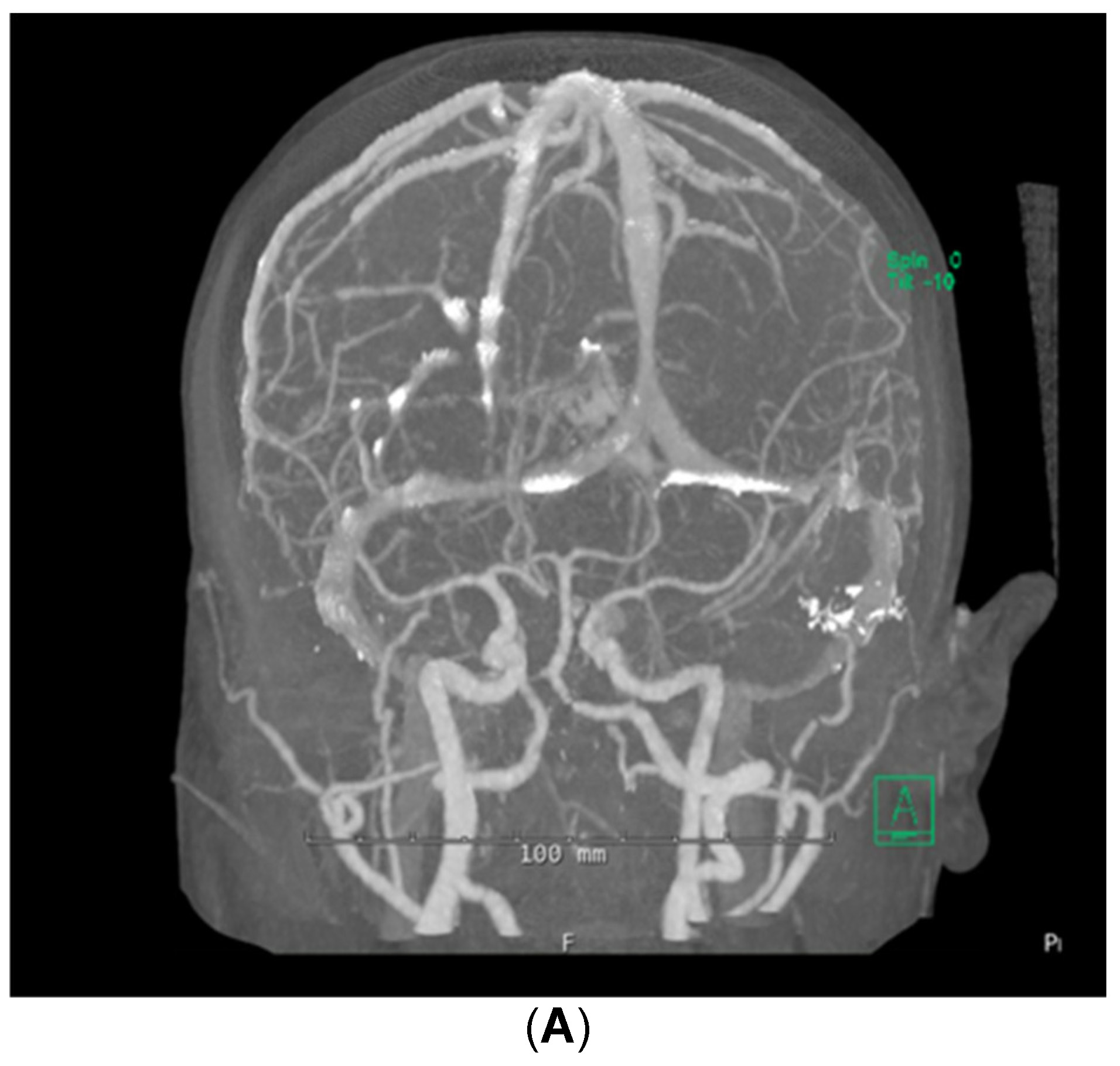
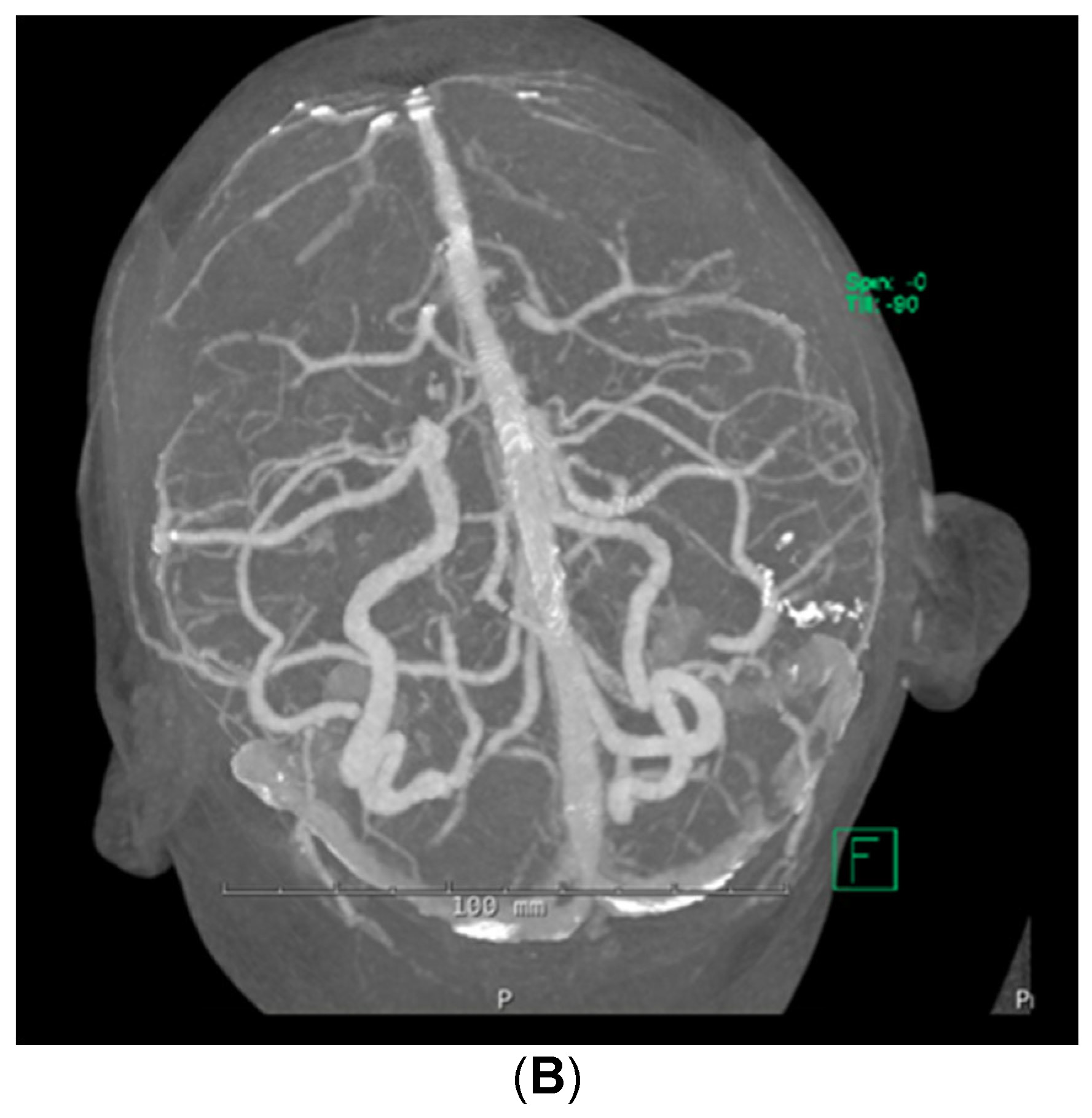
Figure 2: (A,B) CTA brain showing mild irregularities involving M1 segments and right middle cerebral artery bifurcation without vessel occlusions or stenoses within the cerebral vasculature from a posterior (A) and superior-oblique (B) views.
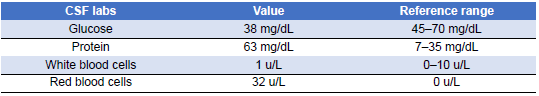
Once the patient’s condition had markedly stabilized and susceptibilities became available (Table 7), carbapenem therapy for Pseudomonas bacteremia was discontinued, and she completed her antibiotic course with oral levofloxacin. The source of the bacteremia was believed to be secondary to bladder translocation in the setting of genital and bladder mucosal ulcerations. Her urine cultures grew the same organism with the same susceptibility pattern. Three days into plasmapheresis treatment, she continued to have fluctuations in cognition despite baseline improvement. Due to the persistence of this waxing and waning level of alertness, a repeat LP was conducted on day 11 of admission (Table 8). The CSF results at that time demonstrated glucose 38 mg/dL (reference range 45–70 mg/dL), protein 63.7 mg/dL (reference range 7.0–35.0 mg/dL), WBC 1 (reference range 0–10 cells/uL), red blood cell count 32 (reference range 0 cells/uL), and no oligoclonal bands were detected. Once again, CSF bacterial and fungal cultures did not demonstrate the growth of any organisms, and polymerase chain reaction (PCR) results for herpes simplex virus and varicella–zoster virus (VZV) were negative as well. Given her time in Ecuador, other diagnostic possibilities were considered, including tuberculous meningitis and Strongyloides hyperinfection syndrome. However, results for tuberculosis CSF PCR, stool ova and parasites, and Strongyloides serologies were negative. She regained full mentation 2 days later while continuing the methylprednisolone and plasma exchange. She became cognitively intact, able to follow commands, and alert and oriented to person, place, and time.
Table 7: Antibiogram Demonstrating Various Susceptibilities of Pseudomonas Aeruginosa
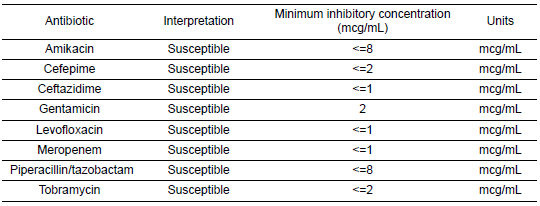
Table 8: Cerebrospinal Fluid (CSF) Analysis on Day 7 of Admission
Based on this patient’s clinical course with improvement on SLE-targeted therapy, the diagnosis of CNSLV was made, and the plan moving forward was to treat the patient with an initial dose of cyclophosphamide of 750 mg followed by monthly infusions, tapering oral prednisone to 50 mg once daily. Trimethoprim–sulfamethoxazole was started as prophylaxis against Pneumocystis jirovecii pneumonia. Hydroxychloroquine 200 mg orally was restarted for SLE, and the patient continued care in the outpatient setting. Two months after this hospitalization, the patient was experiencing mild hair loss due to cyclophosphamide therapy but otherwise was doing well.
Discussion
SLE is an autoimmune condition that may affect the CNS. CNSLV is a manifestation of SLE that can present with neuropsychiatric symptoms [7]. It is typically present in the context of other SLE symptomatology at diagnosis [20]. Furthermore, it should be noted that neuropsychiatric symptoms could occur due to SLE itself, medications, or another comorbid condition [20]. However, before instituting immunosuppressive therapy for CNSLV, it is imperative to rule out infections. Not only are these patients more susceptible to infections, but lupus therapies can severely exacerbate an infectious process and result in life-threatening complications [21].
As infectious diseases consultants, we evaluated this patient and had to comment on the possibility of septic emboli as the cause of her presentation. In addition, we had to comment on the safety of conservative management with antibiotics aimed at an infection or aggressive management with corticosteroids targeting an inflammatory process.
Overall, this patient’s initial presentation stood out for very prominent features of acute SLE that were evident on the exam, including a malar rash, digital ulcerations, arthritis, uveitis, and bleeding oral and genital ulcers. In addition, laboratory workup revealed highly abnormal lupus disease activity markers and signs of visceral involvement, such as hepatitis, carditis, and nephritis. Imaging findings also revealed involvement of the lungs, vasculature, and bladder. In this context, we understood the patient to be in a florid lupus crisis and at high risk for involvement of her CNS.
However, the concurrent Pseudomonas bacteremia posed an additional clinical challenge. Gram-negative rod bacteremia is a serious entity that can lead to septic shock and death, particularly in immunocompromised patients [22]. This patient was highly susceptible to infections and poor outcomes. It was evident that antibiotic therapy was indicated for treating the bacteremia, but whether the bacteremia was causing CNS disease was unclear. We assessed that this patient was at a low risk of endocarditis given the short duration of her bacteremia, the absence of prosthetic materials, and the lack of suggestive findings on transthoracic echocardiography (TTE). We also considered the possibility of preceding nonbacterial thrombotic endocarditis (marantic or Libman-Sacks) as a potential nidus for secondary bacterial endocarditis to develop or as the primary event resulting in metastatic spread to the brain [23]. However, this patient had no apparent primary tumors on the imaging survey and the TTE did not suggest a marantic vegetation. Finally, we understood the organism to be very seldom associated with native valve endocarditis or cerebral septic emboli in a non-IV drug user [24].
Also, under consideration as a source of the bacteremia was Strongyloides hyperinfection syndrome (SHS). The patient was originally from Ecuador and had been there recently, where she could have become infected with the organism. SHS can be associated with overwhelming Gram-negative sepsis, shock, respiratory failure, and death [25]. However, SHS is most associated with chronic steroid use. This patient had not been on any therapy for her SLE, including corticosteroids, and lacked other features, such as peripheral eosinophilia. Other differentials included neurosyphilis, CNS tuberculosis, cryptococcosis, endemic fungal infections, and angioinvasive mold infections, though these were considered less likely and were eventually ruled out. Magnetic resonance venography ruled out septic thrombosis of the cavernous sinuses.
When assessing the brain imaging studies, i.e., MRI, MRA, and MRV we observed a symmetry of cerebral involvement that was very selective to the white matter and a vascular distribution that included both anterior and posterior cerebral vascular territories. Additionally, all lesions appeared to be in the same stage of evolution and showed very similar amounts of perilesional edema with little-to-no rim enhancement. None had undergone central necrosis or showed an air–fluid level. Finally, no mycotic aneurysms were present on MRA or CTA. These findings are atypical for septic emboli in the CNS [26]. There was no meningeal enhancement or sinus disease to suggest a chronic meningitic process or contiguous spread from a parameningeal source.
Further, we found it atypical for abacterial infection to cause such widespread CNS disease without other systemic signs of metastatic infection. These expected findings include florid bacteremia, readily identifiable organisms in the CSF, more cerebral edema on MRI, and appreciable vegetations on echocardiogram. We are aware that TTE has known limitations in sensitivity, and a vegetation that has already embolized may no longer be apparent on a heart valve [27]. Still, the overall constellation of findings pointed away from an infectious etiology and was more consistent with CNS involvement by lupus.
The safest and most appropriate course of action was to continue antibiotic therapy. However, even while this patient was on antibiotics, the possibility of fatal complications was real if we failed to treat an underlying inflammatory process. As she continued to worsen, we decided to initiate high-dose corticosteroid therapy and begin management for presumed CNS lupus involvement. The negative CSF stains and cultures reassured us, but antibiotics were continued.
Once we began therapy for SLE, the patient showed remarkable improvement. At least initially, corticosteroids can also mediate the improvement of infections by reducing inflammation. As more diagnostic data became available, we felt more confident in our diagnosis. Interpretation of the CSF was challenging due to the very low glucose level, which was concerning for infection. However, hypoglycorrhachia is not specific to infection and is reported in association with inflammatory and other noninfectious processes in the CNS [12].
In this case, we consulted with our neuroradiology and neuroimmunology colleagues. The possibility of CNSLV became apparent given the clinical picture, laboratory findings, and suggestive imaging studies. No published American College of Rheumatology diagnostic criteria exist for this rare condition, but the diagnosis can be made based on noninvasive studies [11,16]. While leptomeningeal or cerebral biopsy remains the gold standard, this was not feasible in this patient initially, given her coagulopathy. Once treatment was instituted and the patient improved, we again considered obtaining a biopsy. The yield at this time would have been even lower due to therapy, and the information would not have changed our management approach. Therefore, we decided to forgo a biopsy. Finally, although angiography is essential for diagnosing vascular disease of large- and medium-sized vessels, it cannot detect small-vessel vasculitis [14,19].
For treatment, plasmapheresis and steroids helped the patient initially, and she did well on maintenance therapy consisting of cyclophosphamide and continued steroids [28]. CNSLV therapy can vary but usually includes corticosteroids and plasmapheresis, followed by immunosuppressive agents such as azathioprine and cyclophosphamide [11,16]. Rituximab is a monoclonal antibody that targets the cluster of differentiation (CD)-20 marker on B cells through antibody-dependent cell-mediated cytotoxicity [29,30]. While the synthesis of new antibodies is abrogated, previously circulating antibodies are preserved. This drug has been used successfully as a steroid-sparing agent in CNSLV and lupus cerebritis [31,32]. The prognosis of CNS involvement in SLE can vary, but its morbidity and mortality are higher than in patients without CNS involvement [33,34]. Morbidity in neuropsychiatric lupus stems from strokes, seizures, aseptic meningitis, psychosis, myelopathy, and optic neuritis [35]. Ensuring an early and accurate diagnosis of CNSLV and starting treatment as early as possible is the best way to ensure good patient outcomes.
Conclusions
CNSLV is a rare condition that can result in severe morbidity and mortality. Its presentation can coincide with or mimic infections of the CNS. Therefore, we hope to contribute to the current literature and help infectious diseases clinicians regarding how to approach and navigate such a case. Early
diagnosis and management will minimize adverse patient outcomes.
Author Contributions: Conceptualization, H.G.; methodology, H.G.; software, H.G. and C.S.; validation, H.G. and C.S.; formal analysis, H.G. and C.S.; investigation, H.G. and C.S.; resources, H.G.; data curation, H.G.; writing—original draft preparation, H.G. and C.S.; writing—review and editing, H.G. and C.S.; visualization, H.G. and C.S.; supervision, H.G.; project administration, H.G.; funding acquisition, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aringer, M.; Costenbader, K.H.; Daikh, D.I.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Boumpas, D.T.; Kamen, D.L.; Jayne, D.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [CrossRef] [PubMed]
- Stenszky, V.; Kozma, L.; Szegedi, G.Y.; Sonkoly, I.; Bear, J.C.; Farid, N.R. Heterogeneity of Systemic Lupus Erythematosus Elucidated by Cluster Analysis. Int. J. Immunogenet. 1986, 13, 327–340, Stenszky. Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1744313X.1986.tb01117.x?casa_token=1nwLDXTwLgIAAAAA:NlrFYo0ZpqmDMycU78zawikBPRev4jd_nEDIPNKrGT8YB5grpCK04_omlw382yD53P9IdMxQnXbPD5U (accessed on 13 September 2023). [CrossRef] [PubMed]
- Everett, C.M.; Graves, T.D.; Lad, S.; Jäger, H.R.; Thom, M.; Isenberg, D.A.; Hanna, M.G. Aggressive CNS lupus vasculitis in the absence of systemic disease activity. Rheumatology 2008, 47, 107–109. [CrossRef]
- Jönsen, A.; Bengtsson, A.A.; Nived, O.; Ryberg, B.; Truedsson, L.; Rönnblom, L.; Alm, G.V.; Sturfelt, G. The heterogeneity of neuropsychiatric systemic lupus erythematosus is reflected in lack of association with cerebrospinal fluid cytokine profiles. Lupus 2003, 12, 846–850. [CrossRef]
- Hryb, J.P.; Chiganer, E.; Contentti, E.C.; Di Pace, J.L.; Lessa, C.; Perassolo, M.B. Myelitis in systemic lupus erythematosus: Clinical features, immunological profile and magnetic resonance imaging of five cases. Spinal Cord Ser. Cases 2016, 2, 16005. [CrossRef] [PubMed]
- Leone, P.; Prete, M.; Malerba, E.; Bray, A.; Susca, N.; Ingravallo, G.; Racanelli, V. Lupus Vasculitis: An Overview. Biomedicines 2021, 9, 1626. [CrossRef]
- Rodrigues, M.; Galego, O.; Costa, C.; Jesus, D.; Carvalho, P.; Santiago, M.; Malcata, A.; Inês, L. Central nervous system vasculitis in systemic lupus erythematosus: A case series report in a tertiary referral centre. Lupus 2017, 26, 1440–1447. [CrossRef]
- Fragoso-Loyo, H.; Cabiedes, J.; Orozco-Narváez, A.; Dávila-Maldonado, L.; Atisha-Fregoso, Y.; Diamond, B.; Llorente, L.; Sánchez-Guerrero, J. Serum and Cerebrospinal Fluid Autoantibodies in Patients with Neuropsychiatric Lupus Erythematosus. Implications for Diagnosis and Pathogenesis. PLoS ONE 2008, 3, e3347. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0003347 (accessed on 12 September 2023). [CrossRef]
- Younger, D.S.; Coyle, P.K. Central Nervous System Vasculitis due to Infection. Neurol. Clin. 2019, 37, 441–463. [CrossRef]
- Fang, H.; Lan, L.; Qu, Y.; Zhang, Q.; Lv, J. Differences between central nervous system infection and neuropsychiatric systemic lupus erythematosus in patients with systemic lupus erythematosus. J. Int. Med. Res. 2018, 46, 485–491. [CrossRef]
- Rowshani, A.T.; Remans, P.; Rozemuller, A.; Tak, P.P. Cerebral vasculitis as a primary manifestation of systemic lupus erythematosus. Ann. Rheum. Dis. 2005, 64, 784–786. [CrossRef]
- Chow, E.; Troy, S.B. The Differential Diagnosis of Hypoglycorrhachia in Adult Patients. Am. J. Med. Sci. 2014, 348, 186–190. [CrossRef] [PubMed]
- Castle, J.; Llinas, R.; Wityk, R. Biopsy proven isolated CNS vasculitis, much-vaunted but rarely seen: 20 year retrospective review of brain biopsies. Stroke 2008, 39, 574–574.
- Torres, J.; Loomis, C.; Cucchiara, B.; Smith, M.; Messé, S. Diagnostic Yield and Safety of Brain Biopsy for Suspected Primary Central Nervous System Angiitis. Stroke 2016, 47, 2127–2129. [CrossRef]
- Kim, N.R.; Kang, J.W.; Nam, E.J. Tumor-like Presentation of Cerebral Vasculitis in a Patient with Systemic Lupus Erythematosus: A Biopsy-confirmed Case. J. Rheum. Dis. 2023, 30, 53–57. [CrossRef] [PubMed]
- Nikolov, N.P.; Smith, J.A.; Patronas, N.J.; Illei, G.G. Diagnosis and treatment of vasculitis of the central nervous system in a patient with systemic lupus erythematosus. Nat. Clin. Pract. Rheumatol. 2006, 2, 627–633. [CrossRef]
- Razek, A.A.K.A.; Alvarez, H.; Bagg, S.; Refaat, S.; Castillo, M. Imaging Spectrum of CNS Vasculitis. RadioGraphics 2014, 34. [CrossRef]
- Pomper, M.G.; Miller, T.J.; Stone, J.H.; Tidmore, W.C.; Hellmann, D.B. CNS vasculitis in autoimmune disease: MR imaging findings and correlation with angiography. AJNR Am. J. Neuroradiol. 1999, 20, 75–85.
- Alrawi, A.; Trobe, J.D.; Blaivas, M.; Musch, D.C. Brain biopsy in primary angiitis of the central nervous system. Neurology 1999, 53, 858–860. [CrossRef]
- Ahn, G.Y.; Kim, D.; Won, S.; Song, S.T.; Jeong, H.-J. ; Sohn, I.-W. ; Lee, S.; Joo, Y.B.; Bae, S.-C. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: A prospective, single-center study. Lupus 2018, 27, 1338–1347. [CrossRef]
- Pego-Reigosa, J.M.; Nicholson, L.; Pooley, N.; Langham, S.; Embleton, N.; Marjenberg, Z.; Barut, V.; Desta, B.; Wang, X.; Langham, J.; et al. The risk of infections in adult patients with systemic lupus erythematosus: Systematic review and meta-analysis. Rheumatol. Oxf. Engl. 2021, 60, 60–72. [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, 1974. [CrossRef]
- Characterization of Various Forms of Endocarditis | JAMA | JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/233233 (accessed on 12 September 2023).
- Chittal, A.R.; Rao, S.J.; Lakra, P.; Vietri, R.; Chawla, H. Infective Endocarditis from Pseudomonas aeruginosa and Group C Streptococcus. Cureus 2022, 14, e30904. [CrossRef]
- Geri, G.; Rabbat, A.; Mayaux, J.; Zafrani, L.; Chalumeau-Lemoine, L.; Guidet, B.; Azoulay, E.; Pène, F. Strongyloides stercoralis hyperinfection syndrome: A case series and a review of the literature. Infection 2015, 43, 691–698. [CrossRef] [PubMed]
- Bakshi, R.; Wright, P.D.; Kinkel, P.R.; Bates, V.E.; Mechtler, L.L.; Kamran, S.; Pullicino, P.M.; Sirotkin, I.; Kinkel, W.R. Cranial Magnetic Resonance Imaging Findings in Bacterial Endocarditis: The Neuroimaging Spectrum of Septic Brain Embolization Demonstrated in Twelve Patients. J. Neuroimaging 1999, 9, 78–84. [CrossRef]
- Shively, B.K.; Gurule, F.T.; Roldan, C.A.; Leggett, J.H.; Schiller, N.B. Diagnostic value of transesophageal compared with transthoracic echocardiography in infective endocarditis. J. Am. Coll. Cardiol. 1991, 18, 391–397. [CrossRef]
- Barile-Fabris, L. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann. Rheum. Dis. 2005, 64, 620–625. [CrossRef]
- Zahavi, D.; AlDeghaither, D.; O’Connell, A.; Weiner, L.M. Enhancing antibody-dependent cell-mediated cytotoxicity: A strategy for improving antibody-based immunotherapy. Antib. Ther. 2018, 1, 7–12. [CrossRef] [PubMed]
- Randall, K.L. Rituximab in autoimmune diseases. Aust. Prescr. 2016, 39, 131–134. [CrossRef] [PubMed]
- Choi, S.; Kim, Y.S. Successful treatment with rituximab in a patient with lupus cerebritis and posterior reversible encephalopathy syndrome: A case report. J. Neurocritical Care 2021, 14, 103–108. [CrossRef]
- Tokunaga, M.; Saito, K.; Kawabata, D.; Imura, Y.; Fujii, T.; Nakayamada, S.; Tsujimura, S.; Nawata, M.; Iwata, S.; Azuma, T.; et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann. Rheum. Dis. 2006, 66, 470–475. [CrossRef] [PubMed]
- Fanouriakis, A.; Boumpas, D.T.; Bertsias, G.K. Pathogenesis and treatment of CNS lupus. Curr Opin. Rheumatol. 2013, 25, 577–583. [CrossRef] [PubMed]
- Neuropsychiatric Events in Systemic Lupus Erythematosus: A Longitudinal Analysis of Outcomes in an International Inception Cohort Using a Multistate Model Approach. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31915121/ (accessed on 12 September 2023).
- Hanly, J.G.; Urowitz, M.B.; Gordon, C.; Bae, S.C.; Romero-Diaz, J.; Sanchez-Guerrero, J.; Bernatsky, S.; Clarke, A.E.; Wallace, D.J.; Isenberg, D.A.; et al. Neuropsychiatric events in systemic lupus erythematosus: A longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Ann. Rheum. Dis. 2020, 79, 356–362. [CrossRef] [PubMed]
Figure 1:
MRI T2 axial flair sequence showing numerous punctate to small foci of restricted diffusion with surrounding edema in the supra and infratentorial brain parenchyma. Few of these lesions demonstrate subtle rim enhancement. Mild mass effect without midline shift or other intracranial herniation. A few scattered micro hemorrhagic foci are observed.

Figure 2:
(A,B) CTA brain showing mild irregularities involving M1 segments and right middle cerebral artery bifurcation without vessel occlusions or stenoses within the cerebral vasculature from a posterior (A) and superior-oblique (B) views.


How to Cite: Szewczyk, C.; Gonzalez, H. Enigmatic CNS Lupus Vasculitis: A Heuristic Approach. Priv. Pract. Infect. Dis., 2023, 3(4): 10; doi:10.55636/PPID3040010.

