
Introduction
The association of a skin ulcer (lesion) with regional or distant lymphadenopathy (LAD) has been termed ulcer (U)/node (N) syndrome.
The existence of both adenopathy and ulcerative lesions in a patient can narrow diagnostic possibilities. Appropriate blood and microbiologic exams help confirm the diagnosis.
This syndrome is further classified in one of four categories:
- Predominant lymphadenopathy (N greater than U)
- Lymphadenopathy only
- Predominant ulcerative (U greater than N)
- Ulcer only
1. Predominant Lymphadenopathy
This syndrome is characterized by clinically dominant LAD with a minimal or inapparent ulcer or lesion. The LAD may occur at either solitary or multiple sites.
Two diseases classically fit this category—cat scratch disease (CSD), caused by Bartonella henselae [1], and tularemia, caused by Francisella tularensis. The nodes are typically tender and may be fluctuant. Fever is common in tularemia but less frequent in CSD (Table 1). In contrast, the precipitating lesion may be minimal or clinically inapparent in CSD but is usually apparent in patients with tularemia [2].
Table 1: Cat Scratch Disease and Tularemia Presentation and Treatment.
| Disease | Cat scratch disease | Tularemia |
|---|---|---|
| Organism | Bartonella henselae | Francisella tularensis |
| Fever | Uncommon | Common |
| Incubation | 3–10 days | 3–5 days |
| Lesion Prominence | Less | More |
| Epidemiology | Cats, fleas | Aerosols, cats, rabbits, ticks, muskrats, beavers |
| Therapy | Azithromycin, rifampin, trimethoprim–sulfamethoxazole | For mild disease: ciprofloxacin, doxycycline For severe disease: streptomycin more active than gentamicin |
2. Lymphadenopathy Only
Two diseases classically present with predominant LAD in the absence of clinically apparent ulcers or lesions. Lymphogranuloma venereum (LGV) is caused by Chlamydia trachomatis [3], and plague is caused by Yersinia pestis [4] (Table 2). With both diseases, an initial lesion is present but typically heals by the time a patient presents for evaluation.
Table 2: Lymphogranuloma Venereum and Plague Presentation and Treatment.
| Disease | Lymphogranuloma venereum | Plague |
|---|---|---|
| Organism | Chlamydia trachomatis | Yersinia pestis |
| Fever | Common | Common |
| Incubation | >14 days * | 2–8 days |
| Lesion Prominence | None | None |
| Epidemiology | Sexually transmitted disease | Aerosols, cats, fleas |
| Therapy | Doxycycline, azithromycin | Gentamicin, quinolones |
* Initial lesion is an ulcer with an incubation period ~10 days; this lesion heals and is typically inapparent when the patient presents.
Other diseases that may present with LAD only are acute infectious mononucleosis secondary to Epstein-Barr virus (EBV) and HIV [5]. The LAD is typically generalized in multiple areas and is nontender. With both maladies, patients may also present with lesions. Patients with EBV may experience pharyngitis or, less often, a macular eruption. Patients with HIV may present with a macular eruption early in the disease process.
3. Ulcer Only or U Greater than N
Many diseases present with predominant lesions and a paucity or complete absence of palpable LAD [6] (Table 3). Differential diagnosis includes viruses; condyloma lata, a dermatologic manifestation of syphilis; granuloma inguinale; and cutaneous anthrax. Cutaneous leishmaniasis will also present as a primarily ulcerative process [7].
Table 3: Differential Diagnosis of Only Ulcer/Ulcer Greater Than Node.
| Pathogen | |
|---|---|
| Viruses | Orf Pox: chicken-, cow-, monkey-, small- Herpes simplex virus Molluscum contagiosum Condyloma acuminata |
| Bacteria | Condyloma lata Granuloma inguinale Anthrax |
| Fungi | Blastomycosis, Aspergillus, Mucor |
| Parasite | Leishmania |
| Other | Atypical mycobacteria Syphilis |
Case #1
A 37-year-old photographer returned to the United States from a 6-month trip to East Africa. His primary complaint was painful right inguinal adenopathy for the last several weeks. He had noted an ulcer in that area several weeks prior to the onset of adenopathy. The lesion began as a painless vesicle in the right inguinal region. He experienced low-grade fevers but no chills or weight loss.
He had a long-standing history of well-controlled asymptomatic HIV. He had several unprotected sexual encounters with men while in Kenya.
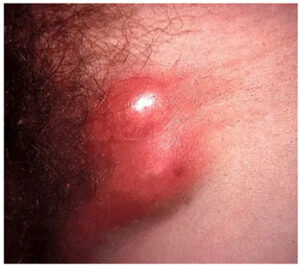
The physical exam revealed prominent matted right inguinal lymph nodes, with a purplish discoloration of the skin and a linear groove (Figure 1) between the matted nodes.

Discussion
The patients history raises concern for sexually transmitted infections (STIs) with ulceration and adenopathy. Since an STI is likely, the differential includes Herpes simplex virus (HSV), syphilis, LGV, chancroid, HIV, monkeypox, and granuloma inguinale. Ulcers associated with pain make HSV, chancroid, and monkeypox more likely. Syphilis and granuloma inguinale are usually painless [9].
The additional presence of prominent adenopathy is seen with chancroid and LGV. The history of a previous ulcer that has resolved and a groove between the nodes (sign of the groove) [10] makes LGV the most likely diagnosis.
Diagnostic lab tests include rapid plasma regain (RPR), drainage culture, and a polymerase chain reaction (PCR) for Chlamydia trachomatis (LGV serovar). Occasionally, a lymph node biopsy is required to make the diagnosis. Given the patients history of travel, sexual encounters, sign of the groove, and a negative RPR, the diagnosis of LGV was likely. A Chlamydia trachomatis nucleic acid amplification test was positive, confirming the diagnosis. The diagnosis of lymphogranuloma venereum could also be proved by a PCR for one of the LGV biovars (L1, L2, L3), if available. The patient was treated with doxycycline 100 mg twice daily for 21 days and was cured of the infection. An alternative therapy, for pregnant women or those intolerant to tetracyclines, is azithromycin 1 gram weekly for three doses.
Case #2
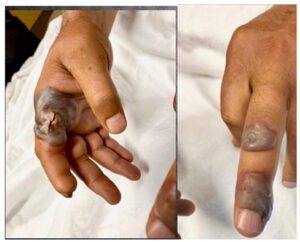
A 51-year-old male presented to the emergency room with a five-day history of small blisters on his right hand and left third finger. The lesions started as small erythematous spots, then progressed in size. No improvement was noted with wound care and topical antimicrobial cream followed by oral clindamycin for seven days. On further history, the patient reported that the lesions started about 5 days after slaughtering a lamb as part of a religious ceremony. Past medical history was unremarkable. The patient had no reported allergies. The physical exam was remarkable for temperature (100 ˝F). Other vital signs were stable. The right medial second finger was notable for a large fluctuant gray blister with surrounding erythema and warmth. Two blisters on the dorsal aspect of the left third finger were present as well (Figure 2). No epitrochlear or axillary adenopathy was appreciated.

Lab results: white blood cell count (WBC), 12,900; glucose, 105; creatinine, 0.71. The aerobic culture of the right-hand blister grew coagulase-negative staphylococci.
Based on the patient history, disease progression, timing, and morphology of the lesions, the diagnosis was orf.
Discussion
Orf is a zoonotic, highly contagious, epitheliotropic DNA poxvirus. Transmission can occur from direct contact with a lesion on a living or deceased animal or through fomites [11].
Diagnosis is based on the appearance of the lesion and exposure history. Histopathology and electron microscopy can demonstrate viral inclusions. Orf virus DNA PCR can be carried out at research labs. The lesions are typically self-limited and do not require active treatment. Lesions generally resolve in 4–8 weeks [12].
Case #3
In late June, a 33-year-old previously healthy male from Missouri presented for an evaluation of a left tender axillary mass, which he had noted over the previous 7 days. He has a cat and several hunting dogs. He hunts and field-dresses the animals.
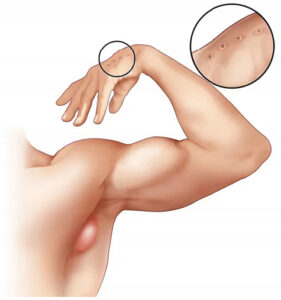
He complained of fever and headache for 3 days. A physical exam revealed a temperature of 102 ˝F with a normal BP and a pulse of 65. A 10 ˆ 7 cm tender fluctuant lymph node was palpated in the left axilla (Figure 3). A papular lesion was also seen on the left first finger.

Labs reveal a WBC of 2800, with a normal differential and normal comprehensive metabolic profile. An x-ray of his finger revealed soft tissue swelling but no osteomyelitis.
Discussion
The differential diagnosis of fever, axillary adenopathy, and a papular skin eruption includes a pyogenic abscess from a staphylococcal or streptococcal species, as well as CSD, tularemia, toxoplasmosis, syphilis, chancroid, tuberculosis, and anthrax. In this case, lymphadenopathy is predominant compared to ulceration.
Cat scratch disease is caused by Bartonella henselae, a facultative intracellular Gram-negative rod, most often transmitted by bites or scratches from a cat. Approximately 30% of cats carry thepathogen, especially kittens. The fleas of cats may also serve as the transmitting agent [14]. Soon after exposure, a mild infection may appear at the skin site. Often, this is associated with a fever, headache, and fatigue. Within 24–48 h, an enlarged lymph node develops upstream of the site of the bite, most commonly in the axilla.
Approximately 14% of CSD cases can have serious systemic complications, including encephalopathy, bacterial endocarditis, hepatosplenomegaly, and inflamed conjunctiva with regional lymph node swelling (Parinauds oculoglandular syndrome) [15].
In this case, the patients contact with wild animals makes tularemia more of a concern. Francisella tularensis, an aerobic Gram-negative rod, causes this zoonotic infection. The papule on the patients finger might have been obtained while hunting. The pulse–temperature deficit, tender adenopathy, and leukopenia make tularemia a prime concern [2].
The diagnosis of tularemia is a real concern for microbiologists. For culturing, it requires a minimum biosafety level 2 environment, and the laboratory should be notified if tularemia is suspected. Blood cultures are less commonly positive than an aspiration of the lymph node. A serologic diagnosis with direct fluorescent antibody or indirect fluorescent antibody tests offer safer and more rapid diagnostic options. These are more useful when carried out 10 days or longer into the illness. PCR tests of blood or tissue could reveal positive results earlier, if available.
This patient responded well to 10 days of ciprofloxacin for the treatment of tularemia. Therapy for mild disease includes ciprofloxacin for 10–14 days or doxycycline for 14–21 days. Severe infections should be treated with streptomycin or gentamicin for 7–10 days.
Case #4
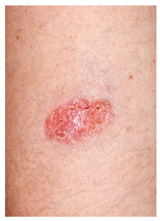
A healthy 59-year-old male went on several excursions through the Amazon jungle. During these trips, he was bitten by numerous insects. He had been vaccinated against yellow fever and was compliant with malaria prophylaxis. Several months ago, he noticed a papule on his right forearm, which ulcerated (Figure 4) and did not heal despite therapy with several antibiotics, antifungal creams, and steroids. More recently, several satellite lesions erupted around the original site. He denies fever, weight loss, neurologic, cardiac, pulmonary, gastrointestinal, or genitourinary issues.

Other than the papule and satellite lesions on his right arm, his physical exam and routine laboratories were all unremarkable. There were no swollen lymph nodes in the right axilla.
Discussion
Leishmaniasis is primarily an ulcerative process. It presents as a chronic, non-healing skin eruption from insect exposure in the tropics (Figure 5).

A more extensive disease that involves the liver and spleen may include anemia, pancytopenia, and hypergammaglobulinemia, also known as kala-azar or visceral leishmaniasis [18]. The diagnosis of leishmania requires the observation of amastigotes in tissue and promastigotes in culture or by a DNA or RNA PCR test. Serological assays in the cutaneous form of leishmania are seldom positive. The treatment for leishmania depends on the specific species and the severity of the infection. There are cutaneous infections, as mentioned above. More serious cases, such as visceral leishmaniasis, typically affect the liver, spleen, and bone marrow.
The patient described was treated with miltefosine for 28 days, which resulted in a positive outcome. Less severe cutaneous infections might be effectively managed with cryotherapy, thermotherapy, intralesional amphotericin, pentamidine, topical paromomycin, and azoles. For patients exhibiting adenopathy, mucosal, or visceral involvement, intravenous amphotericin is usually prescribed.
Conclusion
These cases illustrate how the syndromic approach for patients presenting with various levels of ulceration and lymphadenopathy can narrow in on specific diagnoses. While diseases highlighted in this review may manifest within a spectrum, knowledge of typical presentations and patterns will help clinicians apply further diagnostic tests and, eventually, appropriate treatments.
Author Contributions
Writing—original draft preparation, D.H., R.P., R.F.; writing—review and editing, R.F., R.P, B.H., N.V.H, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tompkins, L.S. Of cats, humans, and Bartonella. N. Engl. J. Med. 1997, 337, 1916–1917. [CrossRef]
- Moriarty, R.A.; Margileth, A.M. Cat scratch disease. Infect. Dis. Clin. North. Am. 1987, 1, 575–590. [CrossRef] [PubMed]
- Bébéar, C.; de Barbeyrac, B. Genital Chlamydia trachomatis infections. Clin. Microbiol. Infect. 2009, 15, 4–10. [CrossRef] [PubMed]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis–etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [CrossRef]
- Morland, B. Lymphadenopathy. Arch. Dis. Child. 1995, 73, 476–479. [CrossRef] [PubMed]
- Mohseni, S.; Shojaiefard, A.; Khorgami, Z.; Alinejad, S.; Ghorbani, A.; Ghafouri, A. Peripheral lymphadenopathy: Approach and diagnostic tools. Iran. J. Med. Sci. 2014, 39 (Suppl. 2), 158–170. [PubMed]
- Machado, P.; Araújo, C.; Da Silva, A.T.; Almeida, R.P.; DOliveira, A., Jr.; Bittencourt, A.; Carvalho, E.M. Failure of early treatment of cutaneous leishmaniasis in preventing the development of an ulcer. Clin. Infect. Dis. 2002, 34, E69–E73. [CrossRef]
- Makama, F.T. Lymphogranuloma Venereum: Health Relevance, Clinical Presentations, Diagnosis and Treatment. HubPages 2002. Available online: https://discover.hubpages.com/health/Lymphogranuloma-Venereum-Health-Relevance-Clinical-Presentations-Diagnosis-And-Treatment (accessed on 9 May 2024).
- Maliyar, K.; Mufti, A.; Syed, M.; Selk, A.; Dutil, M.; Bunce, P.E.; Alavi, A. Genital Ulcer Disease: A Review of Pathogenesis and Clinical Features. J. Cutan. Med. Surg. 2019, 23, 624–634. [CrossRef] [PubMed]
- de Lavaissière, M.; Nougué, J. Lymphogranulomatose vénérienne : Nouveau sérovariant L2b et ancien “signe de la poulie” [Lymphogranuloma venereum: New serovariant L2b and old “groove sign”]. Bull. Soc. Pathol. Exot. 2013, 106, 153–155. [CrossRef] [PubMed]
- Kassa, T. A Review on Human Orf: A Neglected Viral Zoonosis. Res. Rep. Trop. Med. 2021, 12, 153–172. [CrossRef] [PubMed]
- Vellucci, A.; Manolas, M.; Jin, S.; Dwyer, J.; Vick, G.; Wang, A.; Swiatlo, E.; Zheng, C. Orf virus infection after Eid al-Adha. IDCases 2020, 21, e00854. [CrossRef] [PubMed]
- CDC. Cat-Scratch Disease. CDC. Published 2019. Available online: https://www.cdc.gov/healthypets/diseases/cat-scratch.html (accessed on 10 May 2024).
- Higgins, J.A.; Radulovic, S.; Jaworski, D.C.; Azad, A.F. Acquisition of the cat scratch disease agent Bartonella henselae by cat fleas (Siphonaptera:Pulicidae). J. Med. Entomol. 1996, 33, 490–495. [CrossRef] [PubMed]
- Carithers, H.A. Oculoglandular disease of Parinaud. A manifestation of cat-scratch disease. Am. J. Dis. Child. 1978, 132, 1195–1200. [CrossRef] [PubMed]
- LIBRARY DPMP. Cutaneous leishmaniasis Lesion-Stock Image-C038/1507. Science Photo Library. Available online: https://www.sciencephoto.com/media/904178/view/cutaneousleishmaniasis-lesion (accessed on 9 May 2024).
- CDC-Leishmaniasis. Centers for Disease Control and Prevention. Published 2019. Available online: https://www.cdc.gov/parasites/leishmaniasis/index.html (accessed on 10 May 2024).
- Singh, R.K.; Pandey, H.P.; Sundar, S. Visceral leishmaniasis (kala-azar): Challenges ahead. Indian. J. Med. Res. 2006, 123, 331–344. [PubMed]

