Abstract
We report a case of probable neurocysticercosis in a 30-year-old non-Hispanic Caucasian patient without international travel history who presented with breakthrough seizures and a ring-enhancing lesion with perilesional edema. He first developed seizures two years prior, and his workup then demonstrated a single ring-enhancing lesion that was initially attributed to head trauma. On his presentation for breakthrough seizures, his ring-enhancing lesion demonstrated new perilesional edema, and on repeat imaging one month later, near resolution of the cystic lesion. His serum and cerebrospinal fluid serologies for Taenia solium were negative. Of note, the patient reports a friend who developed seizures at around the same time. While this patients demographics, lack of travel history, and negative serologies are not typical of neurocysticercosis in the United States, the characteristic evolution of his cystic lesion from the vesicular to the granular stage on MRI with late-onset perilesional edema and a classical presentation of seizures is most consistent with neurocysticercosis. Considering the absence of other epidemiological risk factors and the occurrence of seizures in close contact during the same timeframe, we hypothesize that the patient was exposed to T. solium through the consumption of contaminated food in the United States.
Case Presentation
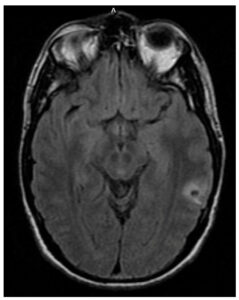
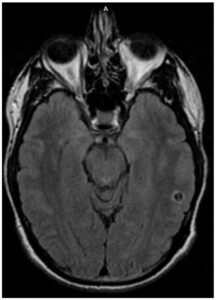
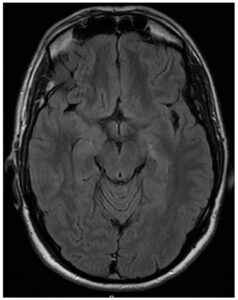
A 30-year-old male with seizure disorder presented for two breakthrough seizures in 2023. His seizure disorder was diagnosed in 2021 when he was started on levetiracetam. He had magnetic resonance imaging (MRI) brain scans performed by his neurologist in 2021 (Figure 1) and 2022 (Figure 2), which demonstrated a single ring-enhancing lesion that was attributed to head trauma during his seizure in 2021. An intracystic enhancement was noted in the 2022 MRI that was possibly consistent with a scolex but, per the patient, his neurologist had ruled out neurocysticercosis (NCC) due to a lack of epidemiological risk factors.


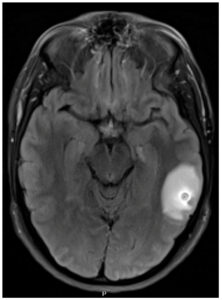
On admission in the spring of 2023, the patient reported a mild headache after his two breakthrough seizures. He denied recent illness, fever, cough, rash, visual disturbances, gait disturbances, or weight loss. The patient is a non-Hispanic Caucasian male born and raised on the East Coast of the United States. He had been working as a middle school teacher for several years. He had no history of overseas travel except for a trip to Puerto Rico in 2022. He had no consistent exposure to live animals. Of note, the patient reported living in San Francisco from 2018 to 2019. He had a friend whom he often dined out with who also developed new seizures in 2021; however, we were unable to contact his friend for further information. On admission, the patient was afebrile without neurological deficits or neck stiffness. His laboratory results demonstrated elevated creatine kinase and therapeutic levetiracetam levels. An MRI of the brain during his admission in 2023 revealed an 8 mm ring-enhancing lesion with regional vasogenic edema (Figure 3). Given the degenerating cyst with significant edema, the decision was made to withhold antiparasitic treatment with close outpatient follow-up. The neurology team increased twice-daily levetiracetam dosage from 750 mg to 1500 mg, which appropriately suppressed further seizures. No corticosteroids were used to control intracranial edema.

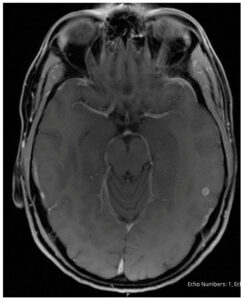
A repeat MRI one month later demonstrated that the cyst had decreased in size to 4.5 mm with total resolution of vasogenic edema, consistent with the granular stage of NCC (Figure 4), and demonstrated near-resolution on repeat MRI three months later (Figure 5). Serum cysticercosis IgG enzyme-linked immunosorbent assay (ELISA), serum and CSF cysticercosis enzyme-linked immunoelectrotransfer blot (EGIB) were negative. Tuberculosis interferon-gamma release assay, cryptococcal antigen, CSF studies including culture and meningitis panel, syphilis screen, Toxoplasma gondii antibodies, Trypanosoma cruzi antibodies and flow cytometry were negative as well.


Discussion
Neurocysticercosis is the most common cause of acquired epilepsy worldwide, and is endemic to countries in Africa, Asia, and Latin America [1]. NCC in the United States affects mainly young adult Hispanic immigrants in the Southwest, with approximate estimates of 0.2 to 0.6 cases per 100,000 individuals in the general population and 1.5 to 5.8 cases per 100,000 among Hispanic people [2]. The primary route of transmission of NCC is through the ingestion of T. solium eggs, which are present in the feces of individuals infected with the adult tapeworm. Cases of transmission have mostly been documented in the setting of travel to an endemic area with contaminated food or water, but have also been documented in the setting of a tapeworm carrier present in the household [2], including housekeepers who may work but not reside in the household [3], and theoretically, in the setting of imported contaminated produce [4]. There was a recent case report of another patient in the United States who developed NCC without travel history or other identified risk factors [5]. The fact that a person who is a close contact of our patient developed a seizure disorder within the same period could signify a potential epidemiological link. Since their primary shared activity was dining out together, it is plausible that they may have been exposed to T. solium through contaminated food in San Francisco. However, our inability to obtain further information from the friend of our patient restricts our ability to draw definitive conclusions.
While neither the patients demographics, exposure history, nor travel history are typical of NCC, the characteristic evolution of his cystic lesion from the vesicular to the granular stage to near-resolution on MRI, and a classic presentation of seizures, is most consistent with NCC. The intracystic lesion detected in the 2022 imaging possibly represents a scolex, which would fulfill an absolute criterion for NCC diagnosis [6]. The patients negative serologies do not definitively rule out NCC; although the sensitivities of EGIB and ELISA tests are approximately 98% when there are more than 2 ring-enhancing lesions, the detection rate for patients with a single lesion or those with nonviable infections is closer to 28% [7,8]. In cases of both single and multiple lesions, antibody detection has been shown to be more sensitive in serum compared to cerebrospinal fluid [7].
NCC is divided into four main neuroradiological stages: vesicular, colloidal, granular, and calcified [9]. Per the Infectious Diseases Society of America (IDSA) guidelines, antiparasitic agents are not recommended for calcified lesions, but they are strongly recommended for viable early-stage NCC. The evidence for treating small solitary nonviable NCC lesions is evolving. Without antiparasitic treatment, approximately 30% to 40% of single NCC granulomas will radiographically clear within a year, while approximately 50% will become calcified lesions [10,11]. In patients with significant cerebral edema, the cystericidal activity of albendazole may worsen edema and increase seizure risk [12]. In patients without significant edema, the IDSA endorses weak to moderate evidence for antiparasitic treatment for patients with nonviable, noncalcified single enhancing lesions [10]. A 2021 Cochrane review cites moderate evidence for antiparasitic treatment compared to placebo for single nonviable, noncalcified intra-parenchymal cysts for seizure reduction (RR 0.61; 95% CI, 0.4 to 0.91; 5 trials, 396 participants), and increased radiological clearance of lesions (RR 1.22; 95% CI, 1.07 to 1.39; 13 trials, 1324 participants) [11]. Overall, there is increasing evidence towards the treatment of nonviable, noncalcified NCC lesions. For our patient, the decision was made to hold antiparasitic treatment given the already significant vasogenic edema triggering breakthrough seizures.
Conclusion
This is a case of probable neurocysticercosis in a non-Hispanic male born and raised in the United States, with no history of international travel, who presented with seizures and a workup that demonstrated an evolving ring-enhancing lesion. Despite negative T. solium serologies, the clinical presentation of seizures and the radiographic progression of the ring-enhancing lesion from the vesicular to granular stage are most consistent with neurocysticercosis.
Author Contributions
Investigation, A.F.Y., M.P. and H.S.; writing—original draft preparation A.F.Y., M.P., Z.A. and H.S.; writing—review and editing, A.F.Y. and H.S.; supervision, H.S.; funding acquisition, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding provided by the Division of Infectious Disease, Allergy, and Immunology at Robert Wood Johnson University Hospital.
Informed Consent Statement
We have received patient consent to include their medical information in a clinical case report.
Acknowledgments
We acknowledge Roy Chowdhury for expertise in neuroradiology.
Conflicts of Interest
No conflicts of interest to disclose.
References
- Gripper, L.B.; Welburn, S.C. Neurocysticercosis infection and disease—A review. Acta Trop. 2017, 166, 218–224. [CrossRef] [PubMed]
- Serpa, J.A.; White, A.C., Jr. Neurocysticercosis in the United States. Pathog. Glob. Health 2012, 106, 256–260. [CrossRef] [PubMed] [PubMed Central]
- Schantz, P.M.; Moore, A.C.; Muñoz, J.L.; Hartman, B.J.; Schaefer, J.A.; Aron, A.M.; Persaud, D.; Sarti, E.; Wilson, M.; Flisser, A. Neurocysticercosis in an orthodox Jewish community in New York City. N. Engl. J. Med. 1992, 327, 692–695. [CrossRef] [PubMed]
- Rivero, L.; Navarro, A. Prevalence of Taenia solium eggs in vegetables for human consumption in Cd. Victoria Tamaulipas Border Epidemiol. Bull. 1986, 14, 1–4.
- Sakhuja, A.; Kc, S.; Wortsman, J.; Shrestha, D.B.; Aryal, B.B.; Kwatra, V.; Verda, L. Severe Neurocysticercosis in an Immunocompetent Male Without Travel to an Endemic Region: A Case Report. Cureus 2023, 15, e34870. [CrossRef] [PubMed] [PubMed Central]
- Guzman, C.; Garcia, H.H. Current Diagnostic Criteria for Neurocysticercosis. Res. Rep. Trop. Med. 2021, 12, 197–203. [CrossRef] [PubMed]
- Wilson, M.; Bryan, R.T.; Fried, J.A.; Ware, D.A.; Schantz, P.M.; Pilcher, J.B.; Tsang, V.C. Clinical Evaluation of the Cysticercosis Enzyme-Linked Immunoelectrotransfer Blot in Patients with Neurocysticercosis. J. Infect. Dis. 1991, 164, 1007–1009. [CrossRef] [PubMed]
- Arroyo, G.; Rodriguez, S.; Lescano, A.G.; Alroy, K.A.; Bustos, J.A.; Santivañez, S.; Gonzales, I.; Saavedra, H.; Pretell, E.J.; Gonzalez, A.E.; et al. Antibody Banding Patterns of the Enzyme-Linked Immunoelectrotransfer Blot and Brain Imaging Findings in Patients With Neurocysticercosis. Clin. Infect. Dis. 2018, 66, 282–288. [CrossRef] [PubMed]
- Sarria Estrada, S.; Frascheri Verzelli, L.; Siurana Montilva, S.; Auger Acosta, C.; Rovira Cañellas, A. Imaging findings in neurocysticercosis. Radiología (English Edition) 2013, 55, 130–141. [CrossRef]
- White, A.C., Jr.; Coyle, C.M.; Rajshekhar, V.; Singh, G.; Hauser, W.A.; Mohanty, A.; Garcia, H.H.; Nash, T.E. Diagnosis and Treatment of Neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 2018, 66, e49–e75. [CrossRef] [PubMed]
- Monk, E.J.M.; Abba, K.; Ranganathan, L.N. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst. Rev. 2021, 6, CD000215. [CrossRef] [PubMed]
- Del Brutto, O.H.; Mera, R.M.; Zambrano, M.; Costa, A.F.; Román, G.C. The Association between Calcified Neurocysticercosis and Cognitive Performance: A Case-Control Study Nested to a Population-Based Cohort. Am. J. Trop. Med. Hyg. 2019, 100, 323–326. [CrossRef] [PubMed]

